Safety, Information, pH Meter
Why & How with pH Meters
What is pH?
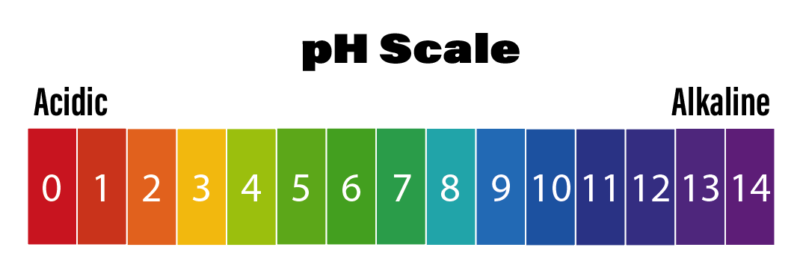
pH is a measurement of how acidic or alkaline a solution is. It is measured in numbers the lower the number, the more acidic the solution. The higher the number the more alkaline the solution.
What is a simple definition of pH?
pH, a quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the hydrogen ion—which ordinarily ranges between about 1 and 10−14 gram-equivalents per litre—into numbers between 0 and 14.
Why maintain the correct pH levels?

The impact of incorrect pH levels can vary from application to application. For example, plants absorb nutrients within a certain pH range so an incorrect level may affect the rate of growth and fruit yield. When brewing, an incorrect pH level can produce a poor quality taste and affect the shelf life. At the other end of the pH scale, a fruit juice producer will have to control pH to avoid poor quality and the risk of causing health issues if the pH drops too low. An incorrect pH level within the pharmaceutical industry could result in producing undesirable toxins. Consistent and precise measurement of pH is fundamental in achieving the result you require.
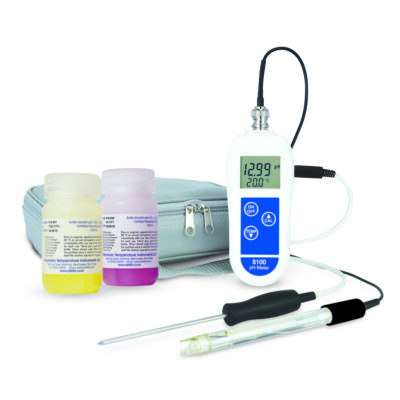
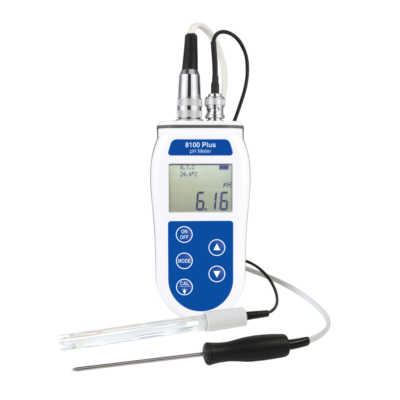
pH Temperature Meter – 8100 Plus Kit
- The robust waterproof case gives IP66/67 protection
- Manual/automatic temperature compensation
- Simple pH re-calibration
- 1-year guarantee
How to Maintain your pH Meter
- If you don’t look after your pH meter then incorrect measurements of pH levels may occur
- As a minimum, you must always clean the pH electrode by holding it under a running tap, if the pH electrode is excessively dirty and a cleaning solution can be used or alternatively use purified water. Leave the electrode in the cleaning solution for at least half an hour, preferably overnight to ensure a thorough clean.
- After soaking overnight, rinse the electrode and then soak in a 4 pH buffer solution before giving the electrode a final rinse. The electrode should then be ready for use.
- When not in use, ensure the pH meter electrode is kept moist in either a storage solution or a 4.01 pH solution. If the sensor is allowed to try out completely, the performance of the instrument will be affected and its guarantee invalidated.
- If an electrode has been allowed to dry out or becomes slow to respond it may be rejuvenated by soaking the electrode overnight in a cleaning solution. Avoid touching the glass bulb at the end of the pH electrode at all times as this can easily cause damage.
Calibrating your pH Meter.

To ensure accurate measurements, it is necessary to calibrate/standardize pH meters on a regular basis. For this, you will require pH buffer solutions. These standard inexpensive solutions are used to check that the pH reading is correct. If it is not, it can be easily corrected by the following procedure for the specific instrument.
Generally, pH electrodes have a limited working life, depending on the frequency of use. this life is approximately twelve months or 365 measurements.
PMI Team – 011 728 6099 – info@pminstrumentation.co.za

